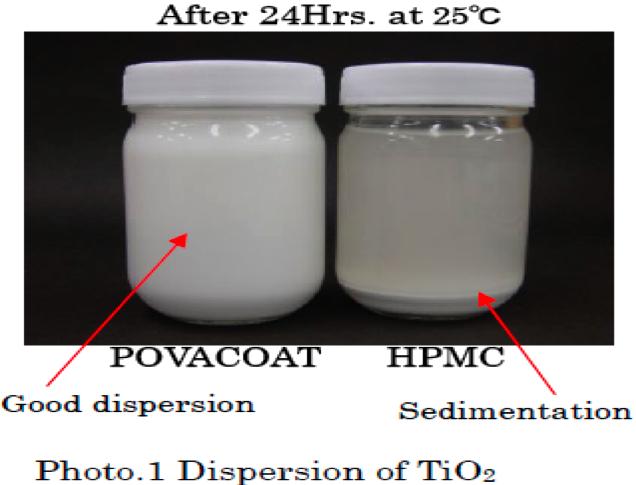
POVACOAT (Polyvinyl alcohol/ Acrylic acid/ Methyl methacrylate copolymer) is a novel pharmaceutical excipient.
Physicochemical Properties
Solubility
- POVACOAT is soluble in water. About 30% of ethanol can be added.
Viscosity
- The linear relationship is shown between viscosity is log and polymer concentration.
Hygroscopic property:
- POVACOAT is hygroscopic and the water content increases with the increase of the relative humidity.
Thermo stability:
- POVACOAT is thermo stable between 130°C and 220°C but degrades above 220°C.
Oxygen Permeability
Permeability of oxygen differs considerably from its competitors and this is a very remarkable characteristic of the POVACOAT membrane. Since POVACOAT can form a dense film resulting from strong hydrogen bonding, the diffusion coefficient of oxygen is much lower than that of other polymer films. As a result, oxygen molecules hardly pass through the film.
Table 1 Results of the oxygen permeability test
Film | Oxygen (mol/m²*sec*Pa) |
|---|---|
Type R | 1.80 x 10^-15 |
Type F | 3.00 x 10^-15 |
Gelatin | 3.35 x 10^-6 |
HPMC | 8.13 x 10^-4 |
Measuring method: manometric visual determination (ASTM D1434)
Measuring condition; 23°C, Film Thickness: 100µm
Strength and elongation of film
The strength for tear of the films decrease with the increase in water content but this decreasing tendency is not so obvious with POVACOAT Type F. With regard to elongation, on the other hand, it can be seen that POVACOAT stretches very well in comparison to other materials.
Table 2 Results of the strength (N/mm²) for tear of the casting films
Film | 25°C/ 50%RH | 25°C/ 75%RH |
|---|---|---|
Type R | 32.2 | 24.2 |
Type F | 25.0 | 20.0 |
Gelatin | 55.6 | 16.7 |
HPMC | 30.8 | 14.7 |
Table 3 Results of the elongation in % of the casting films
Film | 25°C/ 50%RH | 25°C/ 75%RH |
|---|---|---|
Type R | 170 | 312 |
Type F | 50 | 100 |
Gelatin | 5.1 | 11.2 |
HPMC | 5.1 | 3.2 |
Barrier character in film coating
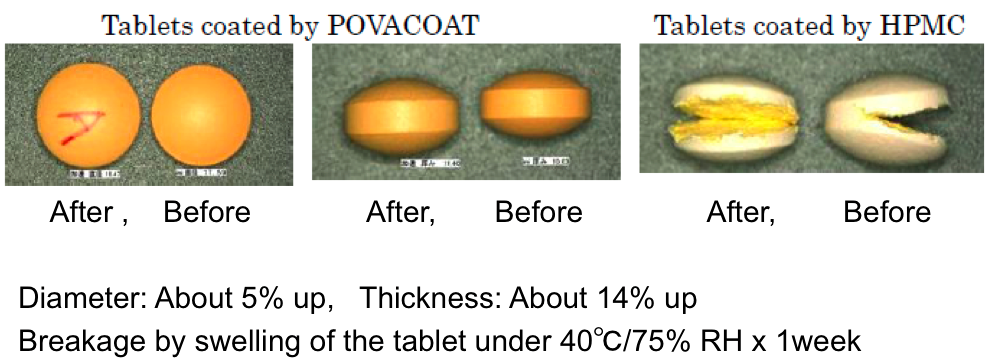
Tablets of OTC drugs combined with sodium chondroitin sulfate sometimes break as a result of swelling due to absorption of moisture. Even for a formulation which breaks with existing HPMC as shown in the photograph, POVACOAT film-coated tablets did not break because POVACOAT stretched to cover swelling of the tablets.
Characteristic
Functional aqueous coating material
- Film formation without plasticizer
- Oxygen barrier effect
(Prevention of oxygenic degradation) - Prevention of whisker or bad smell
- Strong and flexible film
- Prevention of discoloration of tablet printed-ink
Wet granulation growth
- Fast granulation growth
- Broad optimal region for binder liquid amounts
- Granulation based on drug characteristics
(Powder addition or liquid addition) - High tablet hardness under low punching pressure
- Prevention of sticking
Solid dispersion carrier
- Improvement of solubility for hardly soluble drub
- Retardants for re-crystallization
- High stability
- Optimal carrier for solid dispersion with hot-melt extrusion (Thermo-Plasticity)
Products using POVACOAT in Japan |
||
|---|---|---|
New drug (Clinical Trial) | Generic | OTC |
2 Product (phase 3) | 5 products | 9 products |
Film Coating
- Stabilization of drugs which are sensitive to oxygen – – – Oxygen barrier
- Ex.) Ascorbic acid tablet, Macrolide drugs (Clinical Trial)
- Drugs with a bad smell
- Ex.) L-Cysteine/Ascorbic acid tablet, Chinese drug
- Drugs which produce whiskers
- Ex.) Caffeine tablet, ibuprofen tablet
- Prevention of tablet breaking under the high humidity
- Ex.) Chondroitin sulfate tablet
- Moisture resistance for tablet or granule
- Ex.) Applied product is increasing
- Undercoat of sugar coating tablet
- Ex.) Chinese drug
- Prevention of discoloration of tablet printed-ink
Binder
- Wet granulation binder
- High tablet hardness
- Prevention of capping; high content drug (under clinical trial)
- Easily disintegrates
- Orally disintegrating tablet; Ex.) Erythritol or Mannitol OD tablet
- Prevention of sticking
- Ex.) Application now being carried out
- Extruded granules
- High tablet hardness
- Drug layering binder onto core particle
- Dry granulation binder
- Roller compaction
- Direct tabletting
- POVACOAT Type FP (under development)